 Image via Pexels We all strive to live a sustainable life, but sometimes we are not aware that we have the resources to reduce our carbon footprint at our fingertips. In this article, Ask Dr. Ben , partnered with Emma Croft, shows you how to use everyday technology to lead a greener, more sustainable life. These tips are easy to implement and will significantly reduce your environmental impact while saving you money in the long run. Digitize Paper Records as PDF Files Going digital is an efficient way to get rid of paper clutter and store important information electronically. Converting paper records to PDF files not only saves physical storage space, but it also makes document searches quick and easy. However, large PDF files can take up a lot of storage space on your computer or in the cloud. In such cases, trying a PDF compressor can be helpful as it reduces the size of the files without compromising their quality. This makes it easier to store, transfer and access your digitized documents whenever you want. Install a Smart Thermostat Installing a smart thermostat is an excellent way to keep your house comfortable and save energy. Not only will it allow you to control your home's temperature precisely, but it can also learn your schedule and preferences to optimize energy usage. The smart thermostat will adjust the temperature automatically based on the time of day, the weather, and your routine, saving you money on your electric bill and helping the environment. Brighten Up Your Home and Save Money with LED Bulbs If you're looking for a way to brighten up your home while saving money, LED bulbs may be the answer. These energy-efficient bulbs use significantly less energy than traditional incandescent bulbs, which can translate into significant savings on your energy bills over time. Plus, with a longer lifespan than traditional bulbs, you'll have to replace them less frequently, reducing waste and further adding to your savings. By way of comparison, LED bulbs last around 20 years (compared to 5 years for fluorescent bulbs) and consume about 15% of the power of a comparable incandescent (compared to 25% for CFLs). This means LED bulbs make just as much financial sense as environmental sense. They cost a little more up front but quickly pay for themselves via your electric bill. Invest in Solar Panels to Lower Your Energy Bills Investing in solar panels could lower your power bills significantly. While the upfront installation cost is high, you will eventually save on energy costs, and the system will eventually pay for itself. Besides, installing solar panels is an environmentally friendly way to power your home without relying on fossil fuels. Even now, one does need to be careful in considering solar panels for the home from a financial point of view. If you live on the west coast, it’s likely to be a slam dunk in terms of the payoff. But if you live in a part of the country where your electric costs are still running around 11 cents per kilowatt hour, and you maybe don’t get as much sunlight over the year, you very likely will not make your money back unless you’re getting a big break via government subsidy (tax or otherwise). Part of the problem that most people overlook is that solar panels only last 20-25 years and DO require professional inspection and maintenance. Photovoltaics, the supporting inverter electronics and battery systems all come at significant financial and environmental costs. Do the math for your situation, and make sure you buy from a reputable installer. If You Must Own a Car, Get a Hybrid If you live in the US suburbs like me, you simply must have a car. If only to ensure that you get to work on time every morning. Highway vehicles release about 1.5 billion tons of greenhouse gasses into the atmosphere each year. That’s 27% of US emissions. At first glance, you might think that electric cars are the obvious solution, but other factors have to be taken into account. 60.2% of generated electricity is from fossil fuels, one third of that is from coal. Purely electric vehicles also require a great deal of rare earth metals and other environmentally deleterious materials. Hybrids leverage 100+ years of automotive technology and optimization of the internal combustion engine, and marry that efficiency with battery and electric motor technology. At least for now, that marriage of old and new tech, in the appropriate proportions still leads to the most environmentally friendly car option. As with photovoltaics, there will come a day when the new tech is clearly better, but for now we still need to make savvy choices as consumers. Upgrade Your Appliances and Make a Sustainable Impact It's time to say goodbye to your old, unreliable appliances and hello to a sustainable future. By upgrading to energy-efficient models, you can make a positive impact on the environment while also saving money on your utility bills. Not only do these appliances benefit the planet, but they also improve your lifestyle by providing more efficient and effective use of resources. Obviously, be careful about throwing away a perfectly good appliance for one that’s perhaps 10% better in terms of efficiency. Forging the metal, and fabricating a new unit takes a lot of up-front energy as well. But if it’s time for a change anyway, make the smart choice. Some upgrades can improve your environment inside your home as well. Adding a range hood to your kitchen, for example, is one such idea. A range hood helps capture particulate matter generated by cooking, such as oils. This leaves your air cleaner, helping you breathe easier. Use Green DIY Cleaners Switching to DIY cleaners for your home has numerous environmental benefits. For one, homemade cleaners often use natural ingredients, reducing the amount of harmful chemicals that end up in our waterways. These products also tend to be less damaging to the environment as they do not contain phosphates or nitrates, which are common in conventional cleaning products and can contribute to water pollution. Additionally, by making your own cleaners, you reduce the need for plastic packaging, thus cutting down on waste. Lastly, DIY cleaners can be more cost-effective and just as efficient, if not more so, than traditional cleaning products. Downsize Your Living Space Downsizing from a home to a more eco-friendly apartment can be a significant step towards a sustainable lifestyle. Smaller spaces are typically more energy-efficient, reducing your carbon footprint and often leading to lower utility bills. Many eco-friendly apartments also incorporate features like energy-efficient appliances, solar power, water-saving fixtures, and green building materials, further enhancing their environmental benefits. This transition not only supports the environment but can also simplify your life, reducing maintenance responsibilities and often placing you closer to city amenities. Explore your options online if downsizing appeals to you. Adopt Eco-Friendly Business Ideas As people become increasingly environmentally conscious, adopting eco-friendly practices as a business owner can be a major selling point for potential customers. Not only does it demonstrate a commitment to sustainability and reducing carbon footprint, but it also shows that your business cares about the impact it has on the environment. From using recycled materials in packaging to implementing energy-saving measures in the workplace, there are many ways to make eco-friendly changes that appeal to customers. By utilizing everyday technology, we can make a significant impact on the environment and save money at the same time. From DIY cleaners to investing in solar panels, there are numerous ways that we can lead a greener and more sustainable life. If you're a business owner, adopting eco-friendly business ideas will also appeal to customers who are increasingly environmentally conscious. These small changes can lead to significant results and help us all move towards a cleaner, greener future. Understand the Big Picture Finally, if you do want to help make large, political changes then here is my video on the benefits of nuclear power and how it’s likely our only way out in spite of the few incidents we’ve all heard about in the media. Make sure you understand what’s at stake from both sides of this debate and then talk to your representatives.
0 Comments
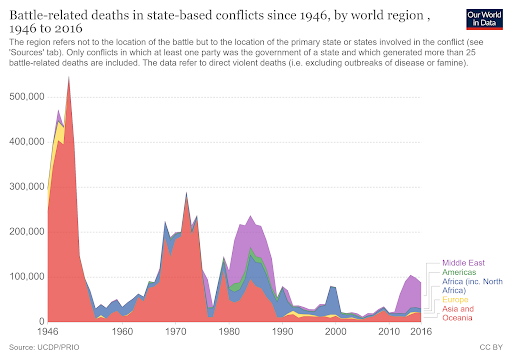
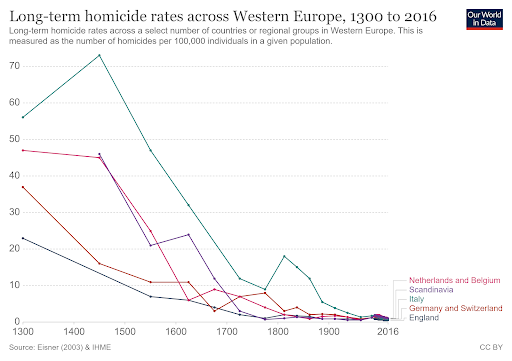
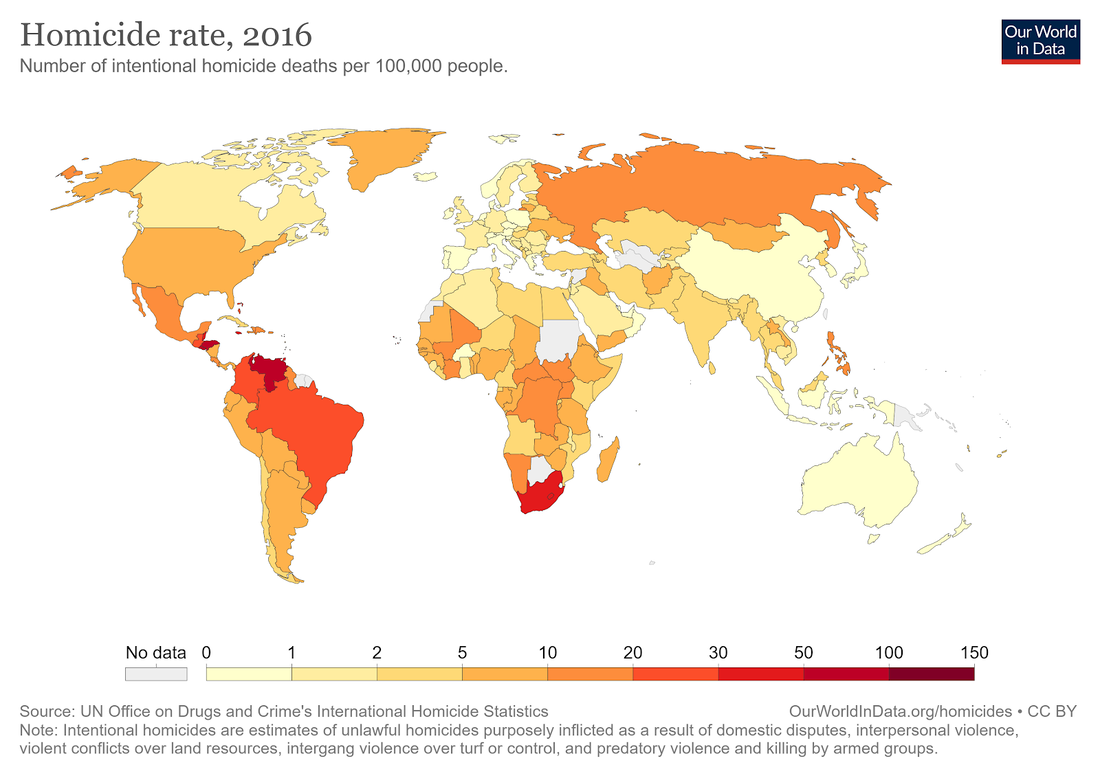
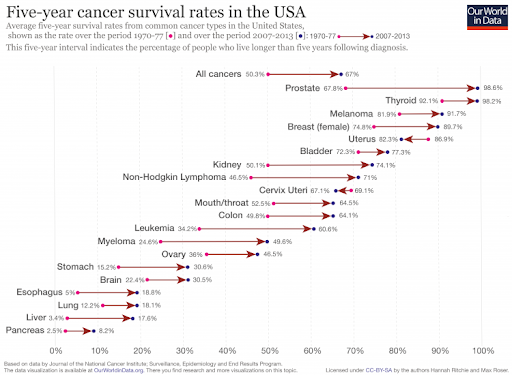
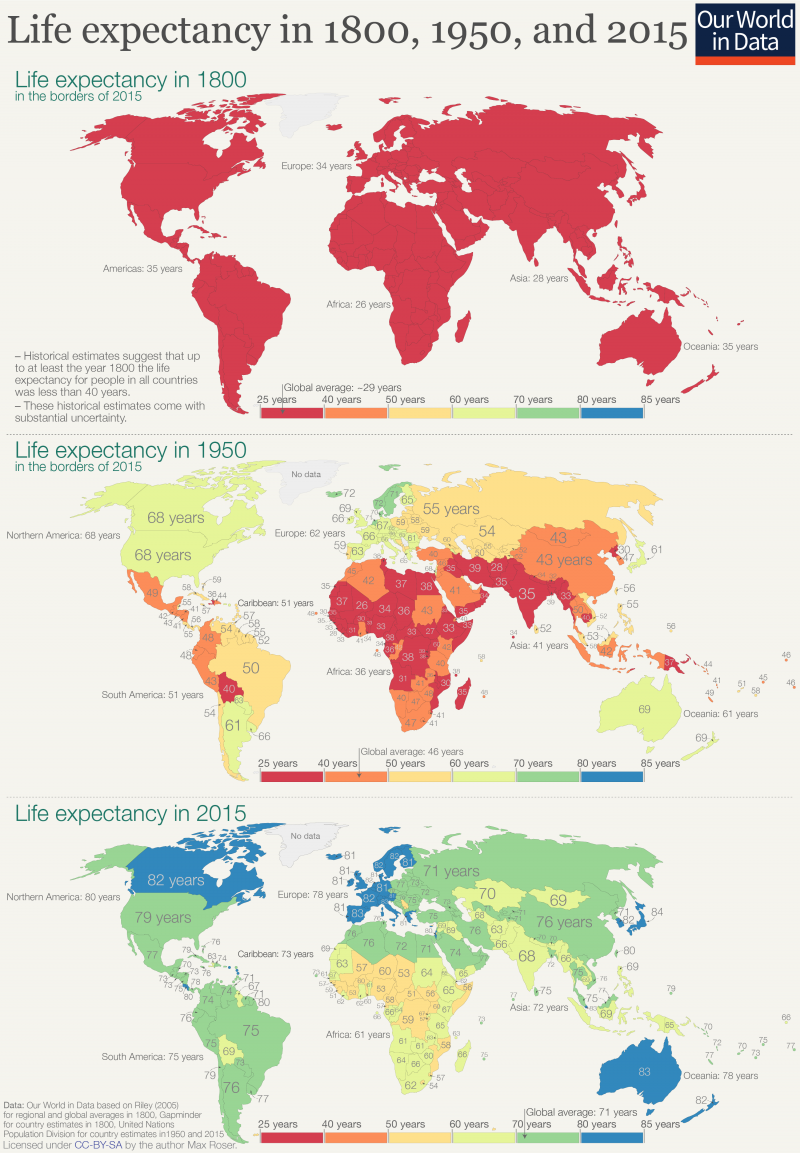
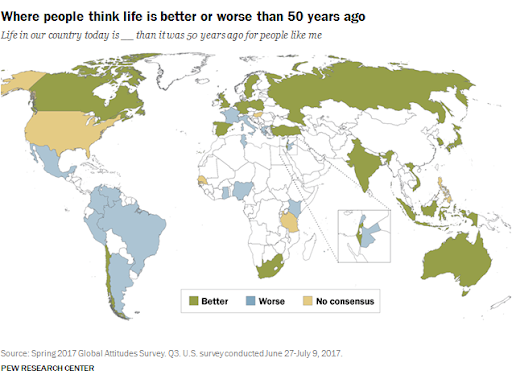
I usually like to think of myself as a purveyor of hope, so about once per year I’ll write an article or give a talk about why the world is NOT ending (or perhaps just steal someone else’s writings). But I’m really not a pollyanna here. I like hope. I see its value. But there’s hope, and then there’s fact. And I’m more than happy to back up what I say with facts. I’m not a good salesman; I can’t seem to convince anyone to do anything, but I can report the facts and let you draw your own conclusions. I can tell you that even though most people obviously think it’s cool and edgy to be cynical about the world, I don’t like what it seems to be doing to our collective psyche. And I don’t feel like it’s productive for any of us. Yes, see the world as it is, but don’t forget to look at the good parts while you’re at it. So, let’s talk first about that which we despise the most: death. Most of us have very immature views on death. Death is a necessary part of evolution. It takes away the ones we love the most (including ourselves in the end), but it makes room for new life and ultimately new paradigms as old standards are replaced in favor of new ones. To hate death is to hate change, but I don’t think anyone really wants to live in a static universe. Now that’s all well and good, so let’s then talk specifically about untimely death, starting with death from war. Here, we see battle-related deaths since the end of WW2. It’s even more impressive when viewed over most of history (and normalized for world population), but the data is less accurate, so we’ll just stick with the last 75 years. https://ourworldindata.org/grapher/battle-related-deaths-in-state-based-conflicts-since-1946-by-world-region If you live in the Middle East, you might have some cause for complaint, but even so, you’re living in Eden compared to the late seventies. With a total max of 100,000, your overall odds of dying in war over the last 20 years are around 0.002 percent. So, OK. If institutionalized violence seems to be on a downward trend, what of individual violence? It seems that, at least pre-Covid, we heard about mass shootings almost weekly. Again, the raw data doesn’t seem to paint the same picture that the six o’ clock news might. https://ourworldindata.org/homicides This particular graph (above) spans 700 years of history (Western Europe). For completeness, let us also look at the relative upstart United States -- its homicide rate -- guns, frontier mentality and all, is 5.35 per 100,000 (2017). That is indeed about five times worse than Europe on average, but it’s still a very small number when viewed historically. https://ourworldindata.org/homicides Most people probably understand that, thanks to medical science, untimely death from disease is on a downward trend as well. Hearing the word “cancer” from an MD was once considered a veritable death sentence, but now, not so much. Because of the wide range of cancer types and the fact that many were never diagnosed prior to death 50 years ago, it’s difficult to describe survival rates with a single graph. However the one below does do a good job of making the case. https://ourworldindata.org/cancer-death-rates-are-falling-five-year-survival-rates-are-rising I’m getting long-winded, so we’ll leave cancer as our proxy for disease in this part of the discussion. Suffice it to say that improvements in medical care have increased survival rates of practically all diseases by around 50 percent over the last 50 years. When you consider that, mathematically, “100 percent” represents an asymptotic limit that can never be crossed, a “half again” improvement on average should truly be viewed as a staggering amount of progress. Personally, I’m also a fan of minimally invasive surgery, which is a byproduct of fiber optics and tool miniaturization. If they can repair a bad heart valve, or remove a tumor without cracking your ribcage open to do it, then you live in a golden age. Finally, there’s an easy way to see if the proof is truly in the pudding here. If the historical causes of death are decreasing (and not being replaced by something else), overall life expectancy should be measurably improving. And here we come to our next exhibit. https://ourworldindata.org/life-expectancy Basically, the above graphic shows that since 1800, for every 50 years of human progress, life expectancy has increased by 10 years or so. Put another way, progress pays us all a 20 percent life bonus for every year we survive. One of my favorite quotes from a history book is from Will and Ariel Durant’s, The Lessons of History (1968): If the prolongation of life indicates better control of the environment, then the tables of mortality proclaim the advance of man, for longevity in European and American whites has tripled in the last three centuries. Fifty years later, undertakers remain unhappy, and progress remains real. An added 1.2 years of life expectancy for every year lived may not quite be a prescription for immortality (even the Tolkien version), but it’s nothing to be sneezed at either. What of quality of life, then? If we’re all just mindless slaves to The Matrix or perpetually impoverished serfs subjugated to the mere 1 percent who have it good, that’s an end of the world scenario of sorts. It’s difficult to find good, overall metrics here, but I did come across an article about a worldwide poll that asked, “Is your country better off now than 50 years ago?” I found the results interesting but not very surprising. Most of Europe and Asia signal with a pretty definite “yes.” The French, being the French, remain perpetually unhappy, and the United States is ambivalent overall. The results of the poll are often best interpreted by the people compiling the data. The following quote speaks volumes to me: While the correlation between economic outlook and positive responses about life today was strong for most countries surveyed, it was not true in all cases — including the United States. "There are countries which, if you look at objectively, are doing well economically but [respondents] still said that life was worse today than it was 50 years ago," says Poushter. "Oftentimes, you see ... more issues with politics, issues with relatively more recent history, in terms of people more upset about where they are compared to 50 years ago." Among U.S. respondents, 37 percent said their lives are better, and 41 percent said they are worse. A lot of that comes down to political divisions, says Poushter. "It's just that in the last year, Republicans have become more likely to say life is better off, and Democrats have become less likely to say their lives are better off," he says. The article goes on to explain that our French (and likely, Italian) brethren have similar angst based solely on the political environment and not their actual quality of life. The problem in South America is an article unto itself, but suffice it to say that it’s understandable why many of them don’t have good feelings about their current situation, particularly Venezuela. But for most of the world, life is better, and they know it. And herein lies my impetus for this article. Many of us have lost perspective due to the groups we immerse ourselves in. It’s always best to remember that MOST people are good at heart. They’re not out to ruin your life. They just have experienced life differently, and they are a jumble of a different set of fears than yours, because sadly, fear is what motivates people the most. We all need to start by remembering that we don’t work 12-hour days fighting to keep food on our table, or the huns at bay. Those battles were fought, and won, on our behalf countless years ago.
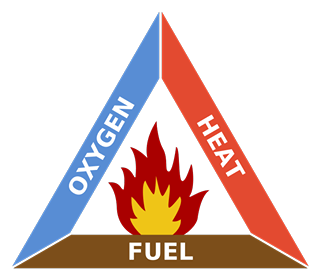
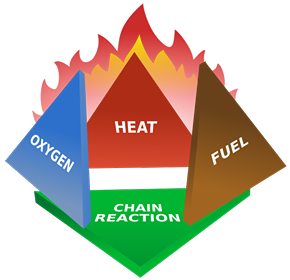
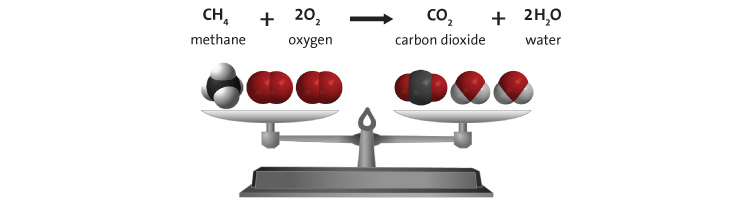
In conclusion, I think part of the problem is that good things tend to happen over time. They’re not dramatic and sudden like a building exploding in Beirut. So it’s natural not to notice goodness as readily as destruction. As Socrates said, "There is only one good, knowledge, and one evil, ignorance." Don’t get so wrapped up in the nightly news, or Facebook, that you become ignorant of our progress, every day, inexorably. Further Reading https://www.wunc.org/post/world-actually-safer-ever-and-heres-data-prove https://www.npr.org/sections/parallels/2017/12/05/568288743/is-life-better-now-than-50-years-ago-the-answer-may-depend-on-the-economy https://www.castlechurchbrewing.com/blog/the-world-is-safer-than-ever-before-so-why-all-the-fear A subscriber recently asked me to explain the physics of fire. I decided since I’ve spent the last year experimenting with explosives that this topic wouldn’t be too much of a stretch for me. The mechanics of fires, large and small, are well understood and generally have been for 100 years or more. But the effects of it are still fascinating to nearly everyone. I’ll try to explain all the interesting aspects of combustion using a candle as our base example. I’ll make sure to insert extra tidbits about roaring campfires and whatnot as I go. The Basics Most everyone is familiar with the concept of the “fire triangle.” The triangle illustrates the three things a fire needs: heat, fuel, and an oxidizing agent (usually oxygen). A fire naturally occurs when these elements are present and combined in something close to the right mixture. Strictly speaking, fire is an event rather than a thing. A fire can be prevented or extinguished by removing any one of the components in the fire triangle. For example, covering a fire with a fire blanket removes the oxygen part of the triangle. In large fires where firefighters are called in, decreasing the amount of oxygen is not usually an option because there is no effective way to make that happen in large volumes of open air. Removing the fuel can be as simple as turning off the gas valve to a Bunsen burner. Removing the heat can be trickier, but one example would be blowing out a candle. The Fire Triangle (https://en.wikipedia.org/wiki/Fire_triangle ) Taking the basic idea further, the fire tetrahedron (below) represents the addition of a component: the chemical chain reaction, to the fire triangle. Once a fire has started, the resulting release of additional energy sets off a chain reaction. Chemical reactions that release more energy than needed to drive them are called “exothermic.” This exothermic reaction sustains the fire and allows it to continue until at least one of the elements of the fire is blocked, or fully consumed. Foam can be used to deny the fire the oxygen it needs. Water can be used to lower the temperature of the fuel below the ignition point or to remove or disperse the fuel. Halon can be used to remove free radicals and create a barrier of inert gas in a direct attack on the chemical reaction responsible for the fire. In the same vein as the fire triangle, as soon as one of the four elements of the tetrahedron is removed, combustion stops. The Fire Tetrahedron (https://en.wikipedia.org/wiki/Fire_triangle ) Combustion is then the chemical reaction that feeds a fire more heat and allows it to sustain itself. When the fire involves burning metals like lithium, magnesium, titanium, etc. (known as a class-D fire), it becomes even more important to consider the energy release. As one counter-intuitive example, these metals react faster with water than with oxygen and thereby release even more energy. Putting water on such a fire results in the fire getting hotter or even exploding. Carbon dioxide extinguishers are ineffective against certain metals such as titanium. Therefore, inert agents (i.e. dry sand) must be used to break the chain reaction of metallic combustion. The Chemistry Modern candles are typically made up of paraffin wax. Paraffin is a fairly complex hydrocarbon chain (or polymer), so let’s start with a simpler example. First, let’s discuss the combustion of methane gas (CH4). This graphic from a middle school chemistry class shows the combustion, or rapid oxidation, of methane. The (balanced) equation for the combustion of methane. (http://www.middleschoolchemistry.com/multimedia/chapter6/lesson1 ) In addition to the idea that methane and diatomic oxygen (O2) rearrange in the reaction to form carbon dioxide (CO2) and water (H2O), the formula is “balanced.” This means that if you count all the atoms on the left side and compare them to the atoms on the left, you’ll see that the numbers are equal. All elements are accounted for, so you know the relative proportions of fuel, oxygen and product. Combustion will occur most efficiently when these relative proportions are present. The fifty cent chemistry word for combining all the components of the reaction efficiently is “stoichiometry.” Another thing worth noting here is methane combustion is smokeless. It’s only products are carbon dioxide and water (both of which are already present in the air). When I started the Science of Explosives talk, I very carefully picked materials that burned without leaving smoke products. This meant I didn’t have to worry about setting off the smoke alarms in the host hotels. So, now knowing all this, let’s move on to the combustion of the paraffin wax (C25H52) in the candle. Here is the balanced chemical reaction: C25H52 + 38 O2 --> 25 CO2 + 26 H2O Although this reaction involves a much larger molecule, we can see that the basic idea is the same. This includes the fact that the candle was should burn cleanly, leaving only water and carbon dioxide behind. Now, anyone that’s had much experience with candles know that they very often leave soot. More on that in a moment, but let’s note a few things. Paraffin is a BIG molecule and it takes 38 diatomic oxygen molecules to fully consume even one paraffin molecule. Just knowing this will hopefully make another fact obvious. This reaction shows the initial components and the final products, but its very likely that it occurs in stages. Put another way it is highly UNLIKELY that 38 O2 molecules jump on a single paraffin chain in one shot. There can literally be over 100 intermediate reactions before a paraffin molecule is completely oxidized. So, we must expect that there will be various combinations of O2 and decomposing paraffin as combustion occurs. This is the key to why candles do not always burn cleanly. The Spark So before talking about the candle, let’s talk about the match. To initiate and sustain combustion, four things are needed: fuel, oxygen, heat and additional heat from an exothermic reaction. Each type of fuel has an ignition temperature, which is the threshold temperature at which that fuel can rapidly unite with oxygen. Put another way, it is the temperature at which the initial molecule has sufficient energy to break apart so that it is free to recombine it’s elements with oxygen. When I was a youngster, Mr. Wizard used to refer to this as the "kindling" temperature. Nowadays, the more common term is called the autoignition temperature (https://en.wikipedia.org/wiki/Autoignition_temperature ). When you light a match, a reaction occurred in the tip of the match that contains potassium chlorate (an oxidizer), sulfur, phosphorus, and starch/glue as binders. 16 KClO3 + 3 P4 S3 --> 16 KCl + 9 SO2 + 3 P4O10 Above, two of the three basic components of the fire triangle are available. All that is needed to light the match is heat. Dragging the match across the rough surface will generate heat due to friction. If the match moves fast enough, friction will generate enough heat and the match will ignite. At the molecular level, this friction will exert enough force to break the molecules apart. In scientific terms, the energy imparted to these molecules will exceed their “activation energy” (https://en.wikipedia.org/wiki/Activation_energy ), or the energy required to start the chain reaction of lighting the match. The phosphorus in the match head has a relatively low ignition temperature—roughly 360 degrees F. Sliding the match head across the rough surface and breaking a few of the chemical bonds, produces a temperature at least this high. Phosphorus is highly reactive and burns quickly, the sulfur is included to sustain the flame long enough to ignite the cardboard (or wood) match stick. Once the phosphorus is ignited, there is plenty of heat energy to autoignite the rest of the match. The Heat Again, for combustion to take place, enough heat needs to be produced by the reactions to keep the temperature of the fuel and oxygen at or higher than the material's ignition temperature. When heat is present, chemical reactions occur because lone hydrogen and oxygen atoms are very reactive; they quickly combine with other molecules or atoms. This leads to a rapid "branching chain reaction." In general, solids and liquids do not burn as a flame—not paper, not even gasoline. What does burn, however, are the vapors that emanate from solids and liquids in the presence of high heat. With a lighted candle, melted wax travels up the wick. When the wax reaches the hot flame along the wick, it vaporizes. The heat from the flame is hot enough to cause the vaporized wax to oxidize (burn), and this oxidation releases more heat. The same thing happens with paper and wood. The heat from the flame is hot enough to vaporize the material, the heated vapor oxidizes, and the oxidizing vapor generates more flame and more heat. So what, then, is a flame? The Flame The continued burning of a candle flame is due to the wicking, or capillary action, of melted wax of melting wax moving through the wick of the candle. As the liquid wax moved into the region of higher temperature, it vaporizes and begins to decompose in the extreme heat. The fancy name for this process is called “pyrolosis” (https://en.wikipedia.org/wiki/Pyrolysis ). A flame has three distinct regions. The innermost zone, directly above the wick, contains wax that has been vaporized but that is unburnt. It is the darkest zone. The middle zone is yellow and luminous. As it is an oxygen depleted zone, insufficient oxygen exists to burn all of the wax vapor. As such, only partial combustion of wax takes place. The zone also contains unburnt carbon vapor. The temperature in this region is hotter than the innermost zone, but cooler than the outer zone. The outer zone is the area where the flame is the hottest and complete combustion of wax takes place. It is light blue in color and not normally visible. The anatomy of a candle flame. (https://en.wikipedia.org/wiki/Candle ) The main determinant of the height of a candle flame is the diameter of the wick. This is evidenced in tealights where the wick is very thin and the flame is very small. Candles whose main purpose is illumination use a much thicker wick. One of Michael Faraday's significant works was The Chemical History of a Candle, where he gave an in-depth analysis of the evolutionary development, workings and science of candles over 150 years ago (https://en.wikipedia.org/wiki/The_Chemical_History_of_a_Candle ). As we saw in the reaction earlier, there are a lot of free carbon atoms released by the decomposition of paraffin wax. Within the flame of the candle, the carbon atoms don't completely combine with the oxygen. Instead, they combine with other surrounding atoms to form large molecules consisting mainly of carbon. These soot molecules get so hot that they glow. The orange-red flame color comes from the glowing soot as it flows into the flame. The glowing soot can be explained in terms of blackbody radiation. This is the effect in physics that explains how and why hot objects emit light and how the colors of that light is a function of the temperature of the glowing material. Blackbody radiation, and the history behind its understanding, is worthy of its own article (https://en.wikipedia.org/wiki/Black-body_radiation ). Unburnt carbon soot emerges from the outside of the flame as smoke particles and eventually deposits on the walls and ceilings of your room as soot. Fire is affected by gravity. Left: Flame on Earth; Right: Flame on the International Space Station. (https://en.wikipedia.org/wiki/Fire ) I've had my Ph.D. for more than twenty five years. One of the most common questions people ask is, "what's the coolest thing you've learned as a scientist?" Well my little plebes, I've decided to pen a list of the ten coolest things I know.  10) Quantum physics still feels to me like there's more to be understood on a deeper level. That being said, particles really aren't solid but "fuzzy" in their behavior (wave-like in certain conditions, point-like in others, depending on scale).  9) Particular to nuclear physics, you can read the age of the earth in the abundance of different radioactive materials in the crust. That was striking to me. It wasn't the original way scientists inferred the age of the earth. It took modern mining and radiometry to provide that confirmation of geologic theory. It’s really satisfying when independent facts come together to form a consistent picture. That’s when you know you’re really on to something.  8) Relativity works. If you accelerate a particle to near the speed of light, its mass and energy really do scale as Einstein predicted. Short-lived particles really do appear to live longer when moving at near the speed of light.  7) Magnetism and magnetic fields aren't real. They're a logical consequence of electrostatics and special relativity because of the moving charges.  6) I have it on good authority (one experiment), that antimatter falls up. So if half the universe turns out to be made of antimatter, it’s still not going to fall towards us.  5) Half of all nuclear power in the US. 10% of the energy market is fueled by re-purposed nuclear missiles once aimed at us.  4) We actually got a positive signal of microbial life on Mars the very first time we looked (Viking II back in 1976). NASA basically refused to believe it could be that easy. The experiment's designer still stands by the results.  3) The long-finned pilot whale has nearly twice as many neurons in it's cerebral cortex than humans (21 b vs. 37 b).  2) Worldwide childhood mortality has been halved since 1990.  1) One scientist - Norman Borlaug is said to have saved more lives (from starvation, up to a billion) than those take by every despot we have numbers for. Have you ever been at a dinner party and someone said “I wish I knew a good analogy describing how electricity flows through wires?” Yeah, me either. But if you ever find yourself in this pesky situation, here’s an article from Ask Dr. Ben. Electrical flow is almost completely analogous to water flowing in a pipe. Pressure is Voltage, current is flow (volume of water, or charge, per second) and resistance is effectively the size of the pipe. A high voltage (pressure) in a big pipe (low resistance) yields a whole lot of flow. Conversely, low voltage in a clogged pipe gets you a trickle. To address wattage (Power = Current x Voltage, after all), just imagine asking how quickly you could pick up a car with a hydraulic piston. More voltage (pressure) and current means you’ll flood the hydraulic chamber and lift the piston faster. This of course assumes DC (“direct current”, or constant voltage), but alternating current (AC) doesn't strain the analogy either. Just imagine a piston/plunger going back and forth in a closed volume. It sucks and blows back and forth but there is definite flow. And flow can do work, even if it needs a check valve or two to push in one direction. Either way, a high resistance restricts how much water you can move. Basic circuit elements are easily modeled too. A capacitor is little more than a tank. A resistor is just a small diameter pipe stuck in line with a larger set of plumbing. A transistor is a pressure switch. An inductor coil is like a little turbine inside the pipe (resists when “spinning up”, but then tries to maintain current flow when being turned off). And yes, a diode is a “check valve”, both are just one-way gates to flow. There’s actually a branch of engineering called Fluidics which leverages this similarity: https://en.wikipedia.org/wiki/Fluidics |
STEM MattersIt seems like in the 50’s and 60’s, “gee whiz” science and technology books and shows abounded. Now, it seems like it’s become trite to most people, old and young. I’d like to reverse that trend in whatever way I can. Technical skills matter. Zeal for science and general understanding of the universe matter. If we lose sight of that as a society, then WE will fail to matter. |




 RSS Feed
RSS Feed
