|
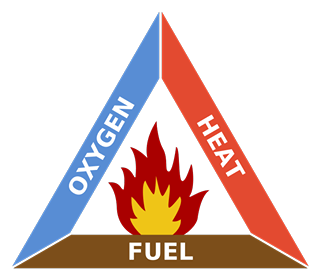
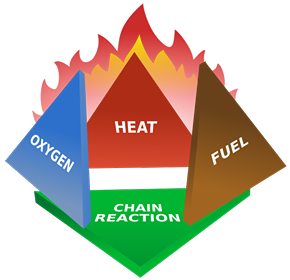
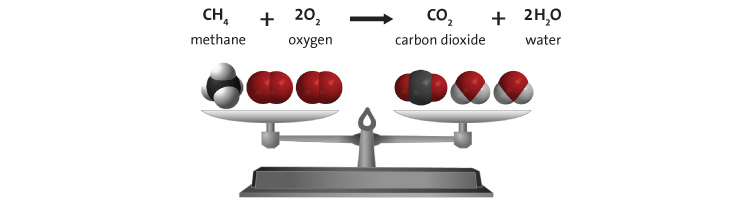
A subscriber recently asked me to explain the physics of fire. I decided since I’ve spent the last year experimenting with explosives that this topic wouldn’t be too much of a stretch for me. The mechanics of fires, large and small, are well understood and generally have been for 100 years or more. But the effects of it are still fascinating to nearly everyone. I’ll try to explain all the interesting aspects of combustion using a candle as our base example. I’ll make sure to insert extra tidbits about roaring campfires and whatnot as I go. The Basics Most everyone is familiar with the concept of the “fire triangle.” The triangle illustrates the three things a fire needs: heat, fuel, and an oxidizing agent (usually oxygen). A fire naturally occurs when these elements are present and combined in something close to the right mixture. Strictly speaking, fire is an event rather than a thing. A fire can be prevented or extinguished by removing any one of the components in the fire triangle. For example, covering a fire with a fire blanket removes the oxygen part of the triangle. In large fires where firefighters are called in, decreasing the amount of oxygen is not usually an option because there is no effective way to make that happen in large volumes of open air. Removing the fuel can be as simple as turning off the gas valve to a Bunsen burner. Removing the heat can be trickier, but one example would be blowing out a candle. The Fire Triangle (https://en.wikipedia.org/wiki/Fire_triangle ) Taking the basic idea further, the fire tetrahedron (below) represents the addition of a component: the chemical chain reaction, to the fire triangle. Once a fire has started, the resulting release of additional energy sets off a chain reaction. Chemical reactions that release more energy than needed to drive them are called “exothermic.” This exothermic reaction sustains the fire and allows it to continue until at least one of the elements of the fire is blocked, or fully consumed. Foam can be used to deny the fire the oxygen it needs. Water can be used to lower the temperature of the fuel below the ignition point or to remove or disperse the fuel. Halon can be used to remove free radicals and create a barrier of inert gas in a direct attack on the chemical reaction responsible for the fire. In the same vein as the fire triangle, as soon as one of the four elements of the tetrahedron is removed, combustion stops. The Fire Tetrahedron (https://en.wikipedia.org/wiki/Fire_triangle ) Combustion is then the chemical reaction that feeds a fire more heat and allows it to sustain itself. When the fire involves burning metals like lithium, magnesium, titanium, etc. (known as a class-D fire), it becomes even more important to consider the energy release. As one counter-intuitive example, these metals react faster with water than with oxygen and thereby release even more energy. Putting water on such a fire results in the fire getting hotter or even exploding. Carbon dioxide extinguishers are ineffective against certain metals such as titanium. Therefore, inert agents (i.e. dry sand) must be used to break the chain reaction of metallic combustion. The Chemistry Modern candles are typically made up of paraffin wax. Paraffin is a fairly complex hydrocarbon chain (or polymer), so let’s start with a simpler example. First, let’s discuss the combustion of methane gas (CH4). This graphic from a middle school chemistry class shows the combustion, or rapid oxidation, of methane. The (balanced) equation for the combustion of methane. (http://www.middleschoolchemistry.com/multimedia/chapter6/lesson1 ) In addition to the idea that methane and diatomic oxygen (O2) rearrange in the reaction to form carbon dioxide (CO2) and water (H2O), the formula is “balanced.” This means that if you count all the atoms on the left side and compare them to the atoms on the left, you’ll see that the numbers are equal. All elements are accounted for, so you know the relative proportions of fuel, oxygen and product. Combustion will occur most efficiently when these relative proportions are present. The fifty cent chemistry word for combining all the components of the reaction efficiently is “stoichiometry.” Another thing worth noting here is methane combustion is smokeless. It’s only products are carbon dioxide and water (both of which are already present in the air). When I started the Science of Explosives talk, I very carefully picked materials that burned without leaving smoke products. This meant I didn’t have to worry about setting off the smoke alarms in the host hotels. So, now knowing all this, let’s move on to the combustion of the paraffin wax (C25H52) in the candle. Here is the balanced chemical reaction: C25H52 + 38 O2 --> 25 CO2 + 26 H2O Although this reaction involves a much larger molecule, we can see that the basic idea is the same. This includes the fact that the candle was should burn cleanly, leaving only water and carbon dioxide behind. Now, anyone that’s had much experience with candles know that they very often leave soot. More on that in a moment, but let’s note a few things. Paraffin is a BIG molecule and it takes 38 diatomic oxygen molecules to fully consume even one paraffin molecule. Just knowing this will hopefully make another fact obvious. This reaction shows the initial components and the final products, but its very likely that it occurs in stages. Put another way it is highly UNLIKELY that 38 O2 molecules jump on a single paraffin chain in one shot. There can literally be over 100 intermediate reactions before a paraffin molecule is completely oxidized. So, we must expect that there will be various combinations of O2 and decomposing paraffin as combustion occurs. This is the key to why candles do not always burn cleanly. The Spark So before talking about the candle, let’s talk about the match. To initiate and sustain combustion, four things are needed: fuel, oxygen, heat and additional heat from an exothermic reaction. Each type of fuel has an ignition temperature, which is the threshold temperature at which that fuel can rapidly unite with oxygen. Put another way, it is the temperature at which the initial molecule has sufficient energy to break apart so that it is free to recombine it’s elements with oxygen. When I was a youngster, Mr. Wizard used to refer to this as the "kindling" temperature. Nowadays, the more common term is called the autoignition temperature (https://en.wikipedia.org/wiki/Autoignition_temperature ). When you light a match, a reaction occurred in the tip of the match that contains potassium chlorate (an oxidizer), sulfur, phosphorus, and starch/glue as binders. 16 KClO3 + 3 P4 S3 --> 16 KCl + 9 SO2 + 3 P4O10 Above, two of the three basic components of the fire triangle are available. All that is needed to light the match is heat. Dragging the match across the rough surface will generate heat due to friction. If the match moves fast enough, friction will generate enough heat and the match will ignite. At the molecular level, this friction will exert enough force to break the molecules apart. In scientific terms, the energy imparted to these molecules will exceed their “activation energy” (https://en.wikipedia.org/wiki/Activation_energy ), or the energy required to start the chain reaction of lighting the match. The phosphorus in the match head has a relatively low ignition temperature—roughly 360 degrees F. Sliding the match head across the rough surface and breaking a few of the chemical bonds, produces a temperature at least this high. Phosphorus is highly reactive and burns quickly, the sulfur is included to sustain the flame long enough to ignite the cardboard (or wood) match stick. Once the phosphorus is ignited, there is plenty of heat energy to autoignite the rest of the match. The Heat Again, for combustion to take place, enough heat needs to be produced by the reactions to keep the temperature of the fuel and oxygen at or higher than the material's ignition temperature. When heat is present, chemical reactions occur because lone hydrogen and oxygen atoms are very reactive; they quickly combine with other molecules or atoms. This leads to a rapid "branching chain reaction." In general, solids and liquids do not burn as a flame—not paper, not even gasoline. What does burn, however, are the vapors that emanate from solids and liquids in the presence of high heat. With a lighted candle, melted wax travels up the wick. When the wax reaches the hot flame along the wick, it vaporizes. The heat from the flame is hot enough to cause the vaporized wax to oxidize (burn), and this oxidation releases more heat. The same thing happens with paper and wood. The heat from the flame is hot enough to vaporize the material, the heated vapor oxidizes, and the oxidizing vapor generates more flame and more heat. So what, then, is a flame? The Flame The continued burning of a candle flame is due to the wicking, or capillary action, of melted wax of melting wax moving through the wick of the candle. As the liquid wax moved into the region of higher temperature, it vaporizes and begins to decompose in the extreme heat. The fancy name for this process is called “pyrolosis” (https://en.wikipedia.org/wiki/Pyrolysis ). A flame has three distinct regions. The innermost zone, directly above the wick, contains wax that has been vaporized but that is unburnt. It is the darkest zone. The middle zone is yellow and luminous. As it is an oxygen depleted zone, insufficient oxygen exists to burn all of the wax vapor. As such, only partial combustion of wax takes place. The zone also contains unburnt carbon vapor. The temperature in this region is hotter than the innermost zone, but cooler than the outer zone. The outer zone is the area where the flame is the hottest and complete combustion of wax takes place. It is light blue in color and not normally visible. The anatomy of a candle flame. (https://en.wikipedia.org/wiki/Candle ) The main determinant of the height of a candle flame is the diameter of the wick. This is evidenced in tealights where the wick is very thin and the flame is very small. Candles whose main purpose is illumination use a much thicker wick. One of Michael Faraday's significant works was The Chemical History of a Candle, where he gave an in-depth analysis of the evolutionary development, workings and science of candles over 150 years ago (https://en.wikipedia.org/wiki/The_Chemical_History_of_a_Candle ). As we saw in the reaction earlier, there are a lot of free carbon atoms released by the decomposition of paraffin wax. Within the flame of the candle, the carbon atoms don't completely combine with the oxygen. Instead, they combine with other surrounding atoms to form large molecules consisting mainly of carbon. These soot molecules get so hot that they glow. The orange-red flame color comes from the glowing soot as it flows into the flame. The glowing soot can be explained in terms of blackbody radiation. This is the effect in physics that explains how and why hot objects emit light and how the colors of that light is a function of the temperature of the glowing material. Blackbody radiation, and the history behind its understanding, is worthy of its own article (https://en.wikipedia.org/wiki/Black-body_radiation ). Unburnt carbon soot emerges from the outside of the flame as smoke particles and eventually deposits on the walls and ceilings of your room as soot. Fire is affected by gravity. Left: Flame on Earth; Right: Flame on the International Space Station. (https://en.wikipedia.org/wiki/Fire )
0 Comments
Leave a Reply. |
STEM MattersIt seems like in the 50’s and 60’s, “gee whiz” science and technology books and shows abounded. Now, it seems like it’s become trite to most people, old and young. I’d like to reverse that trend in whatever way I can. Technical skills matter. Zeal for science and general understanding of the universe matter. If we lose sight of that as a society, then WE will fail to matter. |




 RSS Feed
RSS Feed
